EVYD obtained ISO certification for SaMD
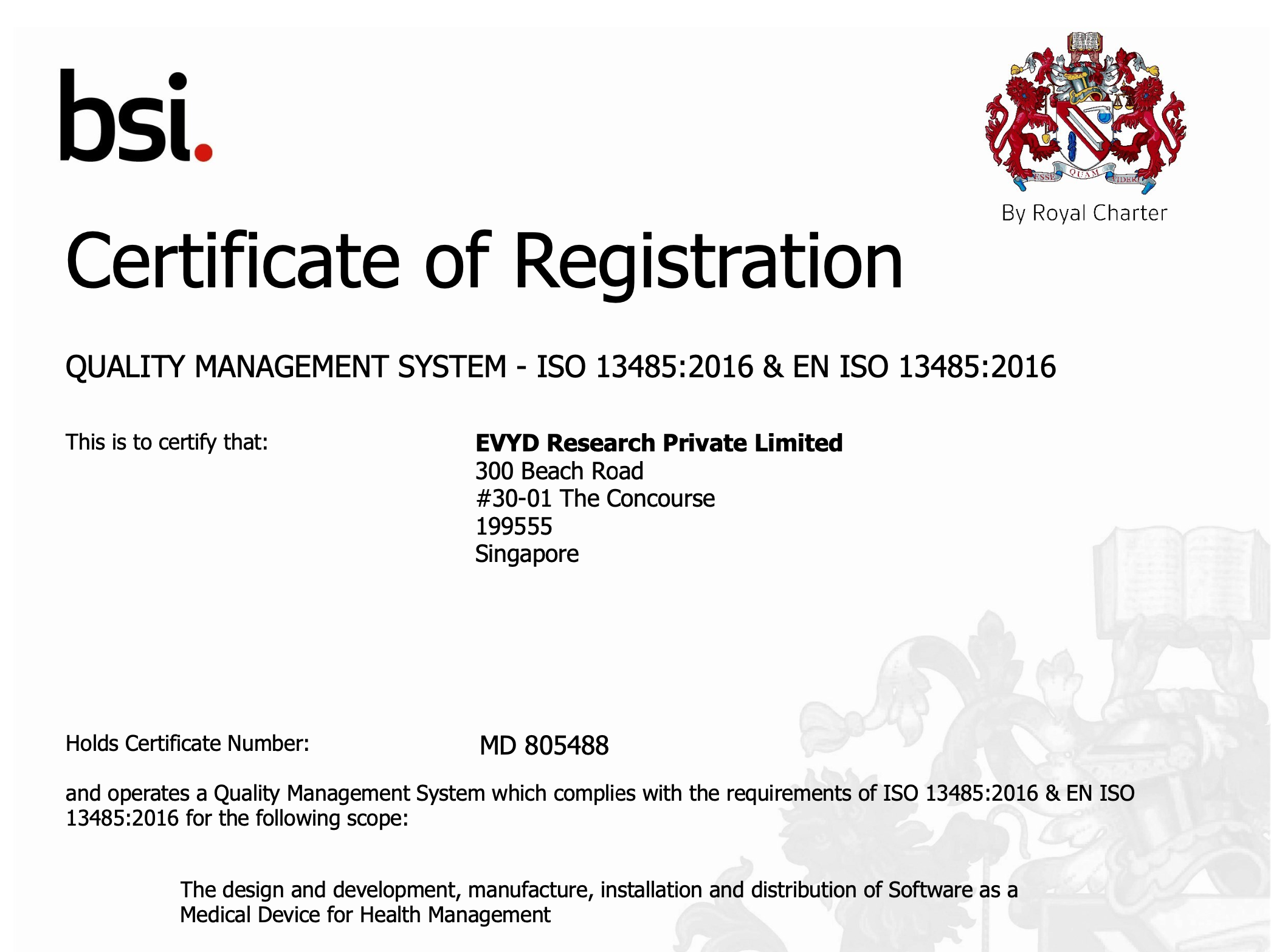
10 June 2024 – EVYD Technology is proud to announce that EVYD has achieved a significant milestone by obtaining ISO certification for the management of the quality of medical devices. We are now officially ISO 13485:2016 and EN ISO 13485:2016 certified for the design and development, manufacture, installation and distribution of Software as a Medical Device (SaMD) for Health Management.
This builds on a recent FDA approval that EVYD worked on to attain for a SaMD designed to support patients aged 21 or older in managing their type 2 diabetes. This software provides real-time feedback to patients and supports treatment plans by healthcare providers. The system analyzes and reports blood glucose test results and encourages medication adherence. In addition, the software provides motivational, behavioral, and educational messages based on blood glucose values and trends. This helps to keep patients engaged and up to date in their knowledge on the condition.
These achievements reflect our unwavering commitment to quality, excellence, and customer satisfaction. We are proud to have undergone rigorous assessments and have been recognized for our ability to meet the international standards set forth by the ISO. Our customers and partners may be assured that our solutions and services adhere to regulatory and international standards.
These milestones would not have been possible without the determination and diligence of our dedicated team. Their pursuit of excellence and drive for quality and continuous improvement have paved the way for these accomplishments.
We are grateful for the support and continued trust from our valued customers and partners and look forward to serving the healthcare ecosystem with dedication and excellence.





















Leave a comment
You must be logged in to post a comment.